
Water, aptly referred to as the ‘elixir of life’, is the primary reason why life exists on our blue planet. It is a key constituent of every living cell, performing multiple roles. From being a medium for essential biochemical reactions, regulating our body temperature, to facilitating the transportation of nutrients within organisms.
However, beyond its apparent physical simplicity, water’s rich chemical properties have intrigued scientists for centuries. This complexity can make water a challenging topic to teach, particularly when explained with reference to chemical properties. For instance, its internal polarity, cohesion and adhesion forces, and surface tension are some abstract nature concepts. They aren’t straightforward for students to comprehend with simple lecturing methods.
This article aims to guide educators and students alike in overcoming these challenges. Our list of five inventive ways will enable learners to appreciate not only the vital role of water in our lives but also the intriguing scientific principles it embodies.
We encounter water in various forms every day, from the morning dew on the grass to the glass of water we drink at dinner. Its physical properties, such as its liquid state at room temperature or the fact it freezes into ice in the winter, are quite familiar to us. However, the internal structure of water, which governs its chemical properties, is often a more complex and abstract concept to grasp.
To elucidate these complexities, engaging and tangible teaching methods are needed. One such method is the use of interactive models. They are a powerful tool for visualizing complex concepts as they enable learners to observe and manipulate the models, offering a multi-sensory learning experience that improves understanding.
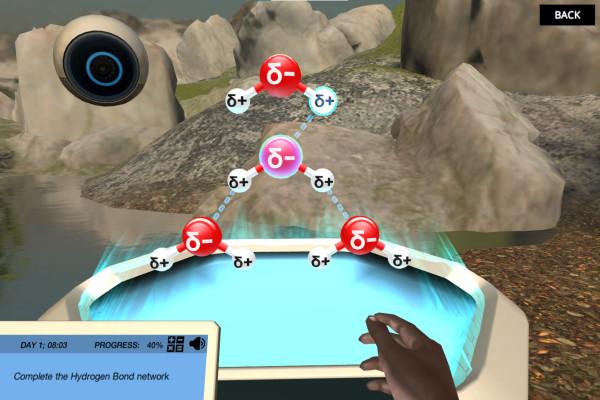
One such example is the Labster Properties of Water Virtual Lab, where students construct their own water molecule on the Holo table. They play around with the molecules, observe the attraction between them, and discover the complex networks formed through hydrogen bonding.
In addition to interactive models, games and activities are another compelling way to make learning enjoyable. Fun learning strategies stimulate curiosity, foster active participation, and promote a positive attitude toward the subject matter, thereby enhancing retention and understanding.
Some interesting activities to foster engagement and participation are:
In the 21st-century classroom, technology plays a pivotal role in revolutionizing the way we learn and teach. With the proliferation of digital tools and resources, educators now have new ways to make complex concepts more engaging, accessible, and comprehensive.
Digital simulations and animations provide dynamic, three-dimensional visualizations that allow students to explore and manipulate water molecules in ways not possible with traditional teaching methods.

For instance, Labster’s Properties of Water simulation is a tech tool that brings the properties of water to life. This simulation helps students visually comprehend abstract concepts such as surface tension. Through strong 3D visuals and interactive models, students can see how water molecules at the surface create a kind of ‘elastic sheet’ due to their cohesive forces, resulting in surface tension.
Discover Labster's Properties of Water virtual lab today!
When educators link classroom lessons to their real-life applications, this provides students with a broader perspective and fosters a deeper interest in the subject.
One way to do this is by connecting the properties of water to career exploration. This approach not only helps students understand the relevance of what they are learning but also exposes them to potential career paths they may not have considered before.
Educators can highlight the importance of this knowledge in various professions. For example, environmental scientists and hydrologists rely heavily on understanding the properties of water as they study water bodies. Similarly, chemical engineers and biochemists often work on manipulating the properties of water in industrial processes or biochemical reactions.
Meteorologists study the role of water in weather patterns and climate change. Moreover, careers in marine biology and oceanography require a deep understanding of water's properties.
Highlighting how the properties of water are used in real-world situations is instrumental in grounding theoretical knowledge with practical reality. This connection demonstrates how concepts learned in the classroom are not isolated, but rather integral to understanding and interacting with our world.
For instance, you can mention how ‘surface tension’, one of water’s properties, allows certain insects to walk on water, facilitates capillary action in plants, and is vital for the process of cleaning in detergents.
Similarly, the polarity of water molecules also has a role in numerous biological processes. For example, it allows proteins and other biological molecules to create specific shapes necessary for their function. Polarity also makes water an excellent solvent, leading to its nickname, the ‘universal solvent’. This property is vital in our bodies where water dissolves nutrients and carries them to cells.
Teaching the properties of water involves more than just presenting facts. It requires engaging students with interactive models, infusing lessons with technology, and connecting theoretical knowledge to real-world applications and careers. Through these strategies, you can make learning an exciting journey of discovery.
Try our free 30-day All Access Educator's Pass today and teach with the Properties of Water simulation alongside 300+ other virtual labs!

Labster helps universities and high schools enhance student success in STEM.
Request DemoRequest a demo to discover how Labster helps high schools and universities enhance student success.
Request Demo